Which Type Of Animal Has A Lateral Line System To Detect Vibrations In Water?
Sound is an important source of information for animals living in the marine surround and all vertebrates, and many invertebrates, have evolved sensory mechanisms for detecting, localizing, and interpreting many of these sounds. The hearing system of vertebrates first arose in fishes, and this group of animals has two contained merely related sensory systems to detect sound. The primary organization is the auditory system (the inner ear), only detection also involves, to a bottom extent, the mechanosensory lateral line arrangement, which is mostly used to find vibration and water flow.
Ane interesting question is why hearing evolved in fishes. While we often think of sound and hearing as of import for advice, it is likely that hearing evolved well before animals could produce sounds to communicate. Instead, it is likely that hearing evolved to help animals use environmental sounds (e.g. waves, rain, underwater geological events, and even sounds from other animals) to learn about their surround.
While the other senses no doubt were likewise plant in primitive fishes, detection of sound provided invaluable added information that helped fishes to survive and thrive. In considering all of the sensory abilities an animal has, it becomes apparent that each sense provides a particular type of information and thus has special roles that enable an animal to survive and thrive in its environment. For example, chemical signals provide data about the presence of other animals or materials in the environment through the odors they emit. However, chemical signals do non provide very adept directional information and work best when the receiving animal is very shut to the chemical source. Similarly, touch is very useful when the fauna is very shut to a stimulus, only not when animals are apart. Vision provides data about objects at greater distances, but it but works if an animal is looking at the object, and it does non piece of work well in depression lite environments or at nighttime.
In contrast, sound provides animals with data about objects at distances beyond that provided by vision and in all directions. In other words, sound provides an brute with a three-dimensional "view" of its earth, and this view is not hindered by currents, light levels, or even the presence of nearly objects (e.k., other organisms, reefs) in the environment.
Indeed, if you think near what senses give you the broadest motion picture of your environment, yous will realize that it is hearing and not vision. While vision is very of import, when yous walk into a dark room you can sense a good bargain about the room from the sounds you hear even if yous can come across cipher. The same goes for fishes and other animals — they go a great deal of information about the "acoustic scene" from their sense of hearing. Thus, in evolving very early on in the history of vertebrates, hearing provided fishes with the ability to larn a great deal about their environment that would non exist bachelor from the other senses. It was only later, as fishes evolved the ability to brand sounds, that hearing became useful for communication, in particular.
The Inner Ear
Fishes are approximately the same density equally h2o, so audio passes correct through their bodies, which motility in concert with the traveling audio moving ridge. Fishes have structures in the inner ear, called otoliths, which are much denser than h2o and a fish's torso. Otoliths are fabricated of calcium carbonate and their size and shape is highly variable among species.

The ear has three otolithic organs and iii semicircular canals. Abbreviations: A, H, P- anterior, horizontal, posterior semicircular canals; AN- auditory nerve to saccule; CC- crus district; L- lagena; LM- lagena macula; LN- eighth nerve to lagena; LO lagenar otolith; Due south saccule; SM saccular macula; And so- saccular otolith; U- utricle; UO- utricular otolith. Copyright © Dr. Arthur N. Popper, Laboratory of Aquatic Bioacoustics, University of Maryland. www.life.umd.edu/biology/popperlab/background/anatomy.htm
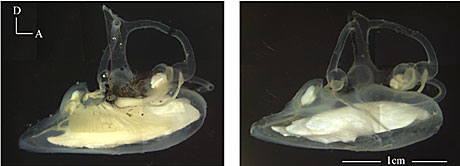
These are pictures of the left and right ears of the blue antimora (Antimora rostrata), a deep-body of water cod. In the picture of the correct ear (on the right), you can clearly meet the 3 otolith organs equally white objects. The saccular otolith in this species is very large and heavy. Copyright © Xiaohong Deng. world wide web.life.umd.edu/biology/popperlab/inquiry/deepsea.htm
Because of the density difference between the fish'south body and the otoliths, the otoliths motility at a dissimilar amplitude and phase in response to sound waves than the rest of the fish. The departure betwixt the motion of the fish's body and the otoliths results in bending of the cilia on the hair cells that are located in the inner ear. This differential move betwixt the cilia and the otolith is interpreted past the brain every bit sound.
Sensitivity to audio, in terms of both the lowest sound levels (loudness or amplitude) and range of frequencies that tin be detected differs among fish species. One cistron affecting the levels and range of frequencies that a fish can hear is the proximity of the swim bladder to the inner ear. The gas within the swim bladder has a density that is much lower than that of seawater and of the fish's torso. As a result, the gas in the swim bladder can be hands compressed by sound force per unit area waves. The swim bladder changes in book in reaction to passing sound waves, essentially re-transmitting the sound stimulus. If the re-transmitted sound from the swim bladder reaches the ear, this may result in the stimulation of the hair cells of the inner ear.
Whether the re-transmitted stimulus from the swim bladder enhances hearing depends on the physical relationship between the swim bladder and inner ear. It appears that species lacking a swim bladder (east.g., elasmobranchs and flatfishes) are not particular sensitive to sounds and accept a narrow hearing bandwidth because they because they do not have any way to detect the sound other than through the inner ear itself.
In contrast, the swim bladder enhances hearing in those species that have structural modifications that help conduct the sound from the swim float to the ear. For example, in the otophysan fishes (e.g., the carps, minnows, catfishes, and characins), the swim float is mechanically linked to the inner ears via the Weberian ossicles, a series of modified bones of the vertebral cavalcade (the start few vertebrae of the backbone). The Weberian ossicles are thought to move in response to sound stimuli that cause movements of the wall of the swim bladder and generally improve hearing sensitivity. For example, goldfish hear up to 3 kHz with best hearing from 500-800Hz.
In other species, the swim bladder may extend forward so that it comes nearly to, or actually contacts, the inner ear. This is constitute in species as various as some squirrelfishes, some butterflyfishes, and in the Atlantic cod. In these cases, when the re-transmitted sound from the swim bladder has to get only a very short distance, so it is more than likely to stimulate the inner ear. Many of these species tin hear sounds higher up ane kHz, and some, like squirrelfish, can hear likewise equally the goldfish and other otophysans.
Finally, fishes that do not have swim float extensions that bring the structure near the ear (e.g., oyster toadfish, tuna, Atlantic salmon) tend to take relatively poor auditory sensitivity, and more often than not cannot hear sounds at frequencies in a higher place 1 kHz. In these species, the re-transmitted stimulus from the swim bladder has little or no touch on hearing capabilities.
One of the most interesting examples of a structural modification that enhances hearing is plant in the clupeiform fishes (e.g., herrings, shads, sardines, anchovies). These fishes take a pair of elongated gas-filled ducts that extend from the swim bladder and enter the skull. Each duct ends in a pocket-sized bubble of compressible gas that comes in contact with a region of the inner ear, the utricle. The presence of a gas bubble in close proximity to the utricle enhances the ability of the swim bladder to stimulate the ear and thus increases hearing sensitivity to a broad range of frequencies. Virtually clupeids tin can observe sounds up to iii-four kHz, which is essentially higher than about marine species which only detect sounds to above one kHz. Moreover, the American shad, equally well as other fishes in the clupeiform family Alosidae, can detect ultrasonic frequencies to over 180 kHz. Behavioral experiments accept shown that when American shad hear ultrasonic clicks like those of dolphins, the fish either swim in the opposite direction of the audio source or motility chaotically, presumably behaviors that would brand it harder for the dolphin to detect and capture the fish.
The Lateral Line System
Fishes have organs called neuromasts on the skin or in hollow, water-filled canals below the peel'southward surface. The neuromasts are found in the head, trunk and tail, and are composed of hair cells that are identical to those of the inner ear. Each pilus cell has a group of cilia on its surface, which are embedded in an overlying gelatinous cupula. The hair cells of a neuromast detect the relative movement of the surrounding h2o relative to the fish. Fishes can use the lateral line organization to discover unidirectional flows and oscillatory flows (vibrations) at short range, over a distance of one to two trunk lengths, and at depression frequencies (0- ~200 Hz).
In the lateral line canals, neuromast organs are spaced out along the length of the culvert, with i organ between two adjacent culvert pores. The pores link the water in the outside environs to the fluid inside the canal allowing changes in the flow field (water motion) effectually the fish to be detected by the neuromasts in the canal. Water movements within the canal, caused by water flows outside the canal, crusade the cupulae to curve alerting the fish to nearby water movement arising from casualty, predators, other members of a school, environmental obstacles, or currents.

3-dimensional interpretation of the lateral line canal ( llc ) on the body of bony fishes. The culvert, which sits at the surface of the fish's body, higher up the trunk musculature ( g ), is contained within a series of overlapping lateral line scales ( s ), which extend from the head to the tail (left to correct in this diagram). The canal is filled with fluid and has pores ( p ) that link the culvert fluid to the external environment. I neuromast receptor organ (n) is found in each of the short tubes within each calibration (two of these tubes are shaded in grey). Movements of water outside of the canal (h2o flows and low frequency vibrations, generated by predators, prey, mates, or environmental sources) cause fluid movement within the canal. The very sensitive neuromasts are composed of ciliated hair cells that are covered by a jelly-like domed cupula, which bends in response to water movements within the canal. The hair cells of the neuromasts are innervated by branches of the posterior lateral line nerve ( plln ), which transmits data about h2o flows to the fish's encephalon. Reprinted with permission from Webb, J. F. and J. B. Ramsay (2017). New interpretation of the iii-d configuration of lateral line scales and the lateral line canal independent within them. Copeia, 105(2): 339-347. American Society of Ichthyologists and Herpetologists (ASIH).
Have you e'er seen fish pond in a schoolhouse? All the fish in the school seem to move exactly in the same direction and as 1 large mass. Equally well as involving vision, the coordinated movements of fishes inside a school are the result of input of water flow information to the lateral line system. As one fish moves in a certain management, it creates a flow of water that provides information to the fish side by side to it or behind information technology. Each fish in the school is able to use this information to accurately maintain their position within a apace moving school.
Additional Links on DOSITS
- Animals > How do fish produce sounds?
- Advanced Content > What components of sound are used for hearing?
- Audio Gallery > Oyster Toadfish
- Sound Gallery > Haddock
- Sound Gallery > Waves
- Audio Gallery > Earthquake
- Audio Gallery > Rainfall
Additional Resources
- Dr. Arthur N. Popper, Aquatic Bioacoustics, Hearing and Audio Detection by Fishes, https://www.ahukini.internet/.
References
- Fay, R. R., & Popper, A. North. (2000). Evolution of hearing in vertebrates: the inner ears and processing. Hearing Research, 149(i–2), 1–x. https://doi.org/10.1016/S0378-5955(00)00168-four
- Mann, D. A., Lu, Z., & Popper, A. Northward. (1997). A clupeid fish can detect ultrasound. Nature, 389 (6649), 341–341. https://doi.org/10.1038/38636
- Plachta, D. T. T. & Popper, A.N. (2003). Evasive responses of American shad (Alosa sapidissima) to ultrasonic stimuli. Acoustics Research Messages Online, 4, 25-30. https://doi.org/10.1121/1.1558376
- Popper, A. N., Fay, R. R., Platt, C., & Sand, O. (2003). Sound Detection Mechanisms and Capabilities of Teleost Fishes. In S. P. Collin & N. J. Marshall (Eds.), Sensory Processing in Aquatic Environments (pp. three–38). New York, NY: Springer New York. https://doi.org/10.1007/978-0-387-22628-6_1
- Popper, A. N., & Hawkins, A. D. (2018). The importance of particle motion to fishes and invertebrates. The Journal of the Acoustical Society of America, 143, 470-486. https://doi.org/ten.1121/1.5021594
- Popper, A. N. and Hawkins, A. D. (2019). An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. Journal of Fish Biology, 94:692-713. https://doi.org/x.1111/jfb.13948
- Popper, A. Due north., Hawkins, A. D., Sand, O., and Sisneros, J. A. (2019). Examining the hearing abilities of fishes. The Journal of the Acoustical Society of America, 146, 948-955. https://doi.org/x.1121/i.5120185
- Schulz‐Mirbach, T., Ladich, F., Plath, Grand. and Heß, G. (2019., Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths. Biological Review, 94: 457-482. https://doi.org/10.1111/brv.12463
- Webb, J. F. (1989). Gross morphology and development of the mechanoreceptive lateral-line system in teleost fishes. Brain, Behavior and Evolution, 33(ane), 34–53. https://doi.org/ten.1159/000115896
- Webb, J.F. (2014a). Morphological multifariousness, evolution, and evolution of the mechanosensory lateral line organisation. In S. Coombs et al. (eds.) Springer Handbook of Auditory Research, The Lateral Line Organisation (pp. 17-72). New York, NY: Springer Science+Business Media. https://doi.org/10.1007/2506_2013_12
- Webb, J.F. (2014b). Lateral Line Morphology and Evolution and Implications for the Ontogenesis of Catamenia Sensing in Fishes. In Flow Sensing in Air and Water. H. Bleckmann et al. (eds.), 247-270.
- Webb, J. F. & Ramsay J.B. (2017). New interpretation of the 3-d configuration of lateral line scales and the lateral line canal contained within them. Copeia, 105(two), 339-347. https://doi.org/10.1643/CG-17-601
- Webb, J. F., Fay, R. R, and Popper, A. N. Eds. (2008). Fish bioacoustics. New York, NY: Springer Science+Business concern Media, LLC.
Source: https://dosits.org/animals/sound-reception/how-do-fish-hear/
Posted by: stopscoperfell.blogspot.com

0 Response to "Which Type Of Animal Has A Lateral Line System To Detect Vibrations In Water?"
Post a Comment